About Forerunner Medical



Founded in 2009, Forerunner Medical group is headquartered in the medical device base in the East District of Zhangjiang Science City, Shanghai. It is a platform enterprise committed to the R & D and production of innovative minimally invasive medical devices; The company has three high barrier core technology platforms: plasma ablation, radiofrequency ablation and implants, and its business covers five fields: sports medicine, spinal minimally invasive surgery, otolaryngology, tumor intervention and respiratory intervention. The company has established a talent team with rich experience in product design, quality control, clinical verification and market development. At present, it has obtained 15 nmpa registrations and more than 100 invention patents; It has also obtained CE product certification and ISO 13485 quality system certification. The core product technology has been applied to more than 1000 hospitals across the country. The core technology products are sold in Europe, Southeast Asia, Australia and other places.
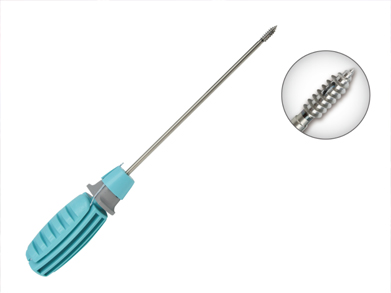
Product introduction
News Center
Forerunner signed a strategic cooperation agreement with Medtronic KangHui
In the national "14th Five-Year plan", new requirements for Department of orthopedics's high quality development were put forward. Meanwhile, with the acceleration of aging in China, the incidence rate of spinal diseases increased year by year.
View more


Two products were approved for listing in one month, and Forerunner Medical transportation platform was quickly built
On July 7, 2021, Forerunner Medical device technology (Shanghai) Co., Ltd. announced that its self-developed orthopaedic implant subdivision product "non absorbable ligament fixing screw" was listed
View more


The 85th China international medical device (Autumn) Expo —— CMEF
The 85th China International Medical Devices (Autumn) Expo - CMEF golden autumn, with clear autumn, was held by Reed Exhibitions group of Sinopharm from October 13 to October 16, 2021
View more


Forerunner Medical implant products have been approved by the State Food and drug administration, creating the first domestic enterprise with a complete product line platform
(Shanghai, China) on June 23, 2021, the official website of China National Drug Administration (nmpa) showed that Forerunner Medical device technology (Shanghai) Co., Ltd
View more